The electrolytic moisture sensor used by Systech Illinois employs the well known phosphorus pentoxide principle for accurate determination of trace levels of moisture in inert gas streams.

Principle of Operation
The method is termed coulometric hygrometry. It is a primary standard and does not need calibration. According to Faradays law of electrolysis the current generated by electrolysis of water can de directly related to its concentration.
The phosphorus pentoxide (P2O5) moisture sensor consists of a dual platinum winding formed around a quartz tube about 8cm long. The bare platinum electrodes are coated with a thin film of P2O5. A constant DC voltage is applied across the windings and the resultant current is monitored. As the gas flows through the cell, the moisture in the gas stream is attracted to the P2O5 coating which is extremely hygroscopic. The moisture migrates through the film to the electrodes (platinum wires) where is it electrolysed. Oxygen is formed at the positive electrode and hydrogen at the negative electrode. The gases diffuse back into the gas stream where they are carried out of the cell. All of the moisture in the gas stream is consumed as it passes through the cell.
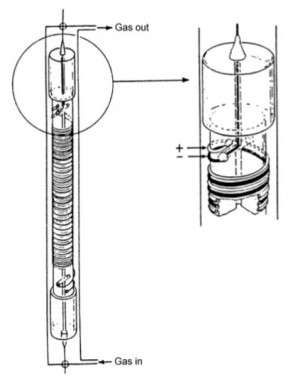
This electrolysis current, according to Faraday’s law, is directly proportional to the amount of moisture in the gas stream. Therefore, a knowledge of the gas flow rate through the sensor and the cell current gives an absolute measure of the moisture contained in the sample gas. The mathematical equation for this measurement is:
I = K1 F C + (e + B )
where:
l = Current
K1 = Faraday’s Constant
F = Mass Flow
C = Moisture Content of Gas
e = Electrolytic Background Current
B = Recombination Current
An effect called recombination can occur if the carrier gas contains hydrogen or oxygen. Recombination occurs because the hydrogen or oxygen in the sample gas stream will combine with the hydrogen and oxygen being produced during electrolysis to form another water molecule. This water molecule is attracted again to the electroydes and electrolysed again, producing a falsely high reading.
Systech Illinois analysers have selectable gas factors which allows compensation of the reading for gases causing recombination.
Cell Recoating
The P2O5 coating on the cells slowly deteriorates over time, especially for high moisture concentrations. Ultimately the cell will no longer respond to moisture and will require recoating. The cell needs to be sent back to the factory for cleaning and recoating. For maximum cell lifetime it is recommended that the cells are purged with clean, dry gas when not in use.
Calibration
Because this measurement principle is a primary measurement method, no calibration is required.
However, it is important to ensure the flow rate of the gas stream remains at the factory setting. Mass Flow Controllers are available for most instruments to ensure the flow rate remains constant.
Applications
Inert gases typically used with this type of instruments include argon, nitrogen, and helium.
The applications for the moisture analyzers fall into two industrial areas:
- Gas Producers: for ensuring product quality
- Gas Users: process gas monitoring (The Pharmacopoeia requires the use of P2O5 for measurements of medical oxygen)
Typical industries are Semiconductor manufacturing, Medical gases, Nuclear processing, and Plastics.
The P2O5 moisture analyzers are not suitable for measurement of moisture in gases or gas mixtures that will react with the phosphorus pentoxide in the detection cell. Such gases include acetylene, butylene, carbon dioxide, hydrogen sulfide, and propylene. In addition, gas mixtures containing compounds which will attack the cell components must be avoided. Systech Illinois can provide special cells with a glass housing which are suitable for measurements in corrosive gases such as chlorine (Cl2) and hydrogen chloride (HCl)
Oxygen and hydrogen cause interference with moisture measurement. Refer to “Recombination” above for further information regarding moisture measurements in these gases.
